
One last option to produce isotopes near the island is to use precise nuclear explosions to make a neutron flux high enough to bypass the gaps of instability at 258–260Fm and at mass number 275 (atomic numbers 104 to 108), imitating the r-process in which the actinides were first formed in nature and the gap of instability around radon bypassed. In recent times it has been observed that the multi-nucleon transfer reactions in collisions of actinide nuclei (such as curium and uranium ) might be used to make the neutron-rich superheavy nuclei situated at the island of stability, although the development of the lighter elements nobelium or seaborgium is more preferred. Other options to create nuclei on the island of stability contain quasifission (incomplete fusion followed by fission) of a massive nucleus, nuclei incline to fission, sacking doubly magic or nearly double magic fragments like as lead-208, calcium-40, tin-132 or bismuth-209. Although the recognized isotopes of moscovium do not really have enough neutrons to be on the island of stability, they can be seen to approach the island as in over-all, the heavier isotopes are the longer-lived. Due to the anticipated high fission barriers, any nucleus within this island of stability completely decays by alpha decay and maybe some electron capture and beta decay. Moscovium is predicted to be in the middle of an island of solidity centered on copernicium (element number 112) and flerovium (element number 114): the reasons for the occurrence of this island, on the other hand, are still not well described. (Image to be added soon) Nuclear Stability and Isotopes About 100 particles of moscovium have been witnessed to date, all of which have been displayed to have mass numbers from 287 to 290. In particular, moscovium should also have important similarities to thallium, as both have one rather roughly bound electron outside a quasi-closed shell. It is measured to have certain assets similar to its lighter homologs, phosphorus, arsenic, antimony, nitrogen, and bismuth, and to be a post-transition metal, while it should also show a number of main differences from them. In the periodic table, it is a member of the 7th period and is placed in group 15 as the heaviest pnictogen, while it has not been established to act like a heavier homolog of the pnictogen bismuth. It is a p-block transactinide element in the periodic table. Moscovium is a tremendously radioactive element: its most stable identified isotope, moscovium-290, has a half-life of only 0.65 seconds. On 28 November 2016, it was publically named after the Moscow Oblast, in which the JINR is placed. In December 2015, it was known as one of four new elements by the Joint Working Party of international scientific bodies IUPAC and IUPAP. It was first manufactured in 2003 by a joint team of American and Russian scientists at the Joint Institution for Nuclear Research (JINR) in Dubna, Russia.
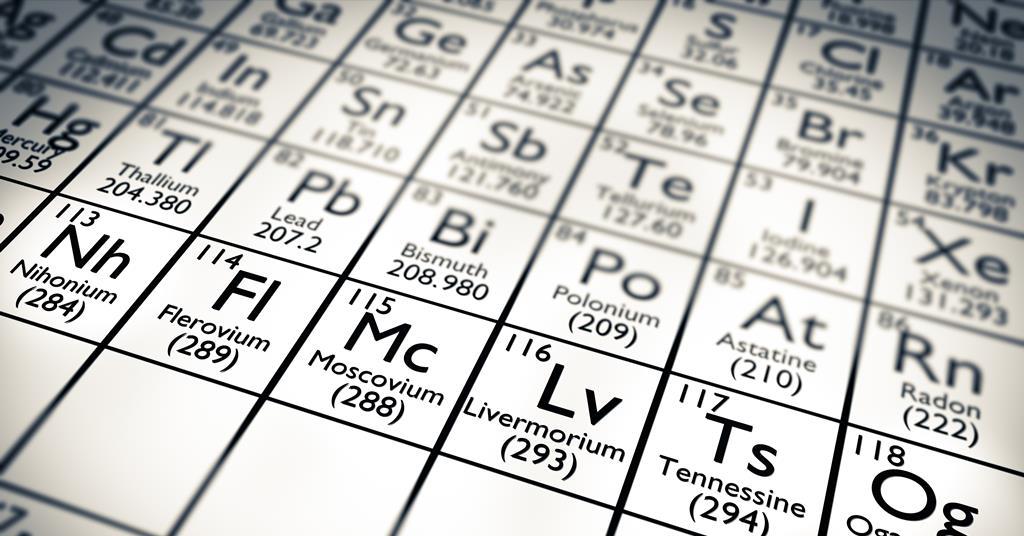

Moscovium is an artificial chemical element with symbol Mc and atomic number 115. Joint Institute for Nuclear Research in 2010


 0 kommentar(er)
0 kommentar(er)
